Sulfate Delivery Methods to Accelerate BTEX Biodegradation and Expedite Site Closure
1. Why Add Sulfate to Impacted Subsurface?
Natural biodegradation of petroleum hydrocarbons is a useful mechanism to reduce concentration and destroy mass of petroleum hydrocarbons in the subsurface. Natural biodegradation begins through metabolism of petroleum hydrocarbons using electron acceptors that are naturally occurring such as oxygen, nitrate, iron III, or sulfate. When these electron acceptors are depleted, petroleum hydrocarbon biodegradation continues to proceed through fermentation reactions that ultimately produce methane. These reactions can be very slow, particularly for benzene biodegradation. Depletion of electron acceptors is common at UST sites, and the slower rates of biodegradation result in persistent high concentrations of benzene, toluene, ethylbenzene, and xylenes (BTEX) in groundwater and persistent source zones of NAPL hydrocarbons in the subsurface. Under these conditions, it is beneficial to overcome these limitations by providing a desirable electron acceptor using an appropriate subsurface delivery method. Of all the naturally occurring electron acceptors, sulfate stands out as the most desirable. It can be applied in large quantities as a solid salt like gypsum, providing a source of sulfate in solution in groundwater that is persistent over time. It can be applied to reach higher target concentrations, which can treat higher concentrations of contaminants in the impacted groundwater.
Sulfate does not adversely affect the flow paths needed to deliver the electron acceptor to the contaminated portions of the aquifer. It does not promote biofouling and the reaction products have a small volume. Sulfate-reducing conditions are ubiquitous in the presence of petroleum hydrocarbons, and sulfate is often a depleted electron acceptor, which underlines its active participation in petroleum hydrocarbons biodegradation processes. A comparative summary of natural electron acceptors is provided in Table 1.
Table 1: Summary of Soluble Electron Acceptor Advantages and Concerns (adapted from Cunningham et al., 2001; Sra et al. 2022b).
| Reaction | Electron Acceptor (Reactant) | Maximum Concentration in Water (mg/L) | Benzene Consumed (mg/L) | Notes / Likely Issues |
| Aerobic oxidation | O2 | 9 | 3.0 | Limited solubility. Numerous other oxygen sinks. Potential aquifer clogging. Biofouling near injection point. |
| Nitrate reduction | NO3‑ | 45 | 9.5 | Drinking water concern. Primary MCL 10 mg/L NO3‑-N (45 mg/L NO3‑).Expensive. |
| Sulfate reduction | SO42- | 250 | 55 | Hydrogen sulfide is the product, but rarely an issue due to precipitation with iron in soil or sediment. Secondary MCL for sulfate – 250 mg/L. Much cheaper than nitrate. |
1.1 When is Sulfate Addition Most Suitable?
- Sustained concentrations of petroleum hydrocarbons in groundwater that prevent achieving groundwater clean-up objectives and/or site closure and depleted sulfate concentration in plume / source zone compared to background.
- Absence of measurable LNAPL in the monitoring well(s) within the target area for sulfate addition at a site. Target areas at a site with residual LNAPL (i.e., without measurable LNAPL in a monitoring well) can be considered for sulfate addition.
1.2 Key Benefits of Appropriate Site Application
Addition of sulfate in petroleum hydrocarbon-impacted subsurface where sulfate is depleted in groundwater:
- Provides a cost-effective and sustainable alternative to mechanical remediation, oxygen release-based biodegradation (e.g., use of oxygen release compounds, ORC) and in-situ chemical oxidation (ISCO). Sulfate addition enhances biodegradation of petroleum hydrocarbons and can address the more depleted portions of the source area (fringes of the source), thereby reducing the area/volume requiring active remedial alternatives such as excavation (Kolhatkar and Schnobrich, 2017).
- Can reduce time to closure (and enable cost savings in reduced long-term groundwater monitoring costs). At sites with high benzene concentrations (e.g., >1,000 μg/L) considered indicative of residual LNAPL source, a decrease in benzene concentration will likely be observed after concentrations of other hydrocarbons have decreased following sulfate application.
1.3 Chevron Experience With Sulfate to Promote Enhanced Biological Degradation
As of 2023, Chevron has performed sulfate delivery at nine sites including one former terminal site, four former retail sites, one former chemical storage facility, two former refinery sites, and one operating refinery. Evaluation and/or remedial action is underway at four more sites (two oil field sites and two refinery sites).
For wells at these sites, long term monitoring data were analyzed to calculate attenuation rate constants for benzene before sulfate addition (kMNA for conditions of “monitored natural attenuation” or MNA) and after sulfate addition (kSEA for conditions of “sulfate-enhanced attenuation” or SEA). The rate constants were estimated as the slopes of a linear regression of the natural logarithm of benzene concentration on the date of sampling. Example data from two wells are presented in Figure 1.
In the well depicted in Panel A of Figure 1, the addition of gypsum increased the attenuation rate constant for benzene from 0.55 per year to 1.6 per year. Starting at 10,000 µg/L benzene in 2006, it took 4.2 years to get to 1,000 µg/L and would take another 10 years to reach the MCL of 5 µg/L under conditions of MNA, attaining the goal by 2020. Starting at 1,000 µg/L in 2011, it took 3.3 years to the MCL under conditions of sulfate-enhanced attenuation, attaining the goal by 2015. In the wells depicted in Panel B of Figure 1, the initial concentration of benzene was very high (5,500 µg/L). Prior to the addition of sulfate, there was no evidence for attenuation of benzene or the TEX compounds (toluene, ethylbenzene, and xylenes). Even though the concentration of benzene was high, the addition of sulfate resulted in a substantial rate constant for attenuation of benzene and an even more substantial rate constant for the attenuation of the TEX compounds.
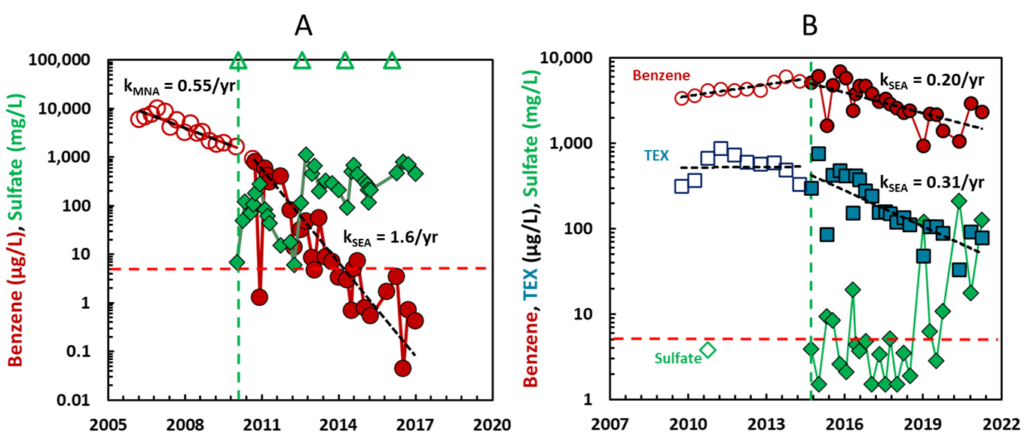
Figure 1 summarizes data from 31 wells at the nine sites subjected to sulfate addition using gypsum land application (29 wells) and permeable filled borings (two wells). In many of the wells prior to sulfate addition and for a few of the wells after the addition of sulfate, there was an increasing trend in benzene concentrations, or the attenuation rate constant was essentially the same as zero. In these cases, we arbitrarily assigned a low, non-zero value to kMNA or kSEA (0.03 per year), to allow us to estimate a lower boundary on the time required to reach the MCL for benzene. Many of the wells in Figure 2 with > 100 years of clean-up time fall in this category. The pre-sulfate benzene concentrations range from 20 to 9,500 μg/L with 16 wells having pre-sulfate benzene concentrations > 1,000 μg/L considered indicative of residual LNAPL conditions.
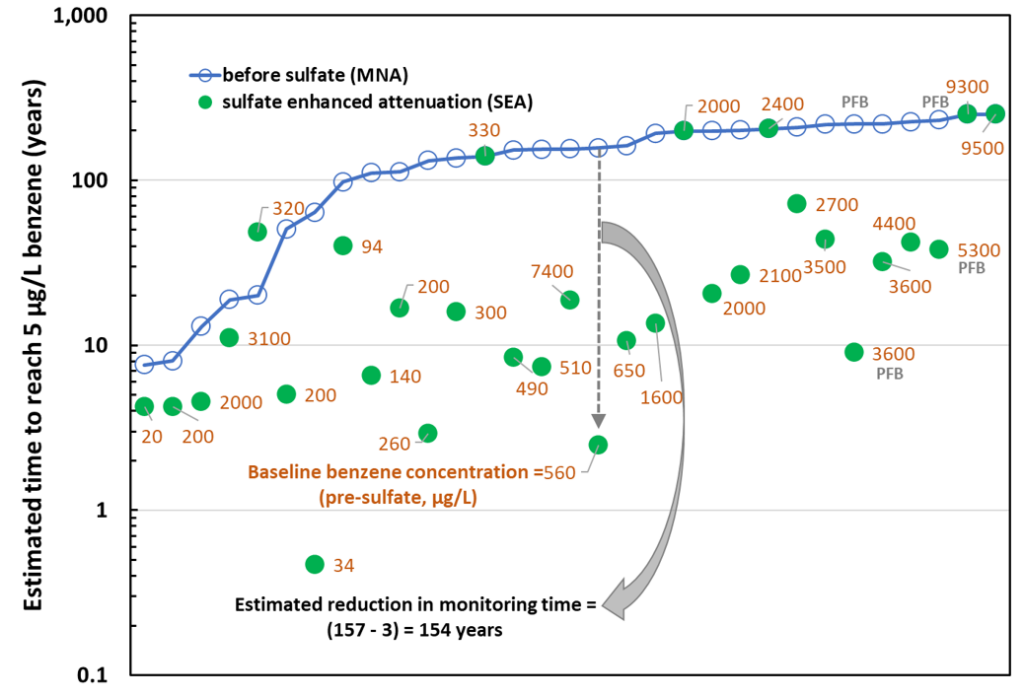
The gray dashed arrow and solid gray arrow in Figure 2 illustrate the significant decrease in the timeframe to reach the MCL for one particular well. The timeframe decreased from an estimated 157 years to three years marking a change of 154 years. Overall, sulfate addition at sites depleted with sulfate significantly improved timeframe to cleanup from a median of 150 years (for MNA) to 15 years (for sulfate-enhanced attenuation through sulfate addition).
At these monitoring wells the gypsum land application and permeable-filled borings resulted in sustained sulfate breakthrough, induced sulfate-reducing conditions, and enhanced degradation of BTEX. Petroleum hydrocarbon degradation through sulfate reduction was also monitored through CSIA of 13C and 2H in benzene, 34S in SO42- and 13C in DIC in groundwater which had been depleted in sulfate prior to sulfate addition.
Based on the experience and results from these sites, sulfate addition to impacted subsurface with depleted sulfate has significantly enhanced the rate of BTEX biodegradation and improved site outcomes (e.g., optimize excavation footprint, expedite site closure).
2. What Sulfate Delivery Method is Suitable for My Site?
Table 2 presents a summary of different sulfate delivery strategies that are recommended, with the corresponding preferred site conditions. Recommended strategies include land application, permeable filled borings, permeable trench, and excavation backfill.
Note that injection or placement of sulfate slurry (or solution) into wells has been shown to be ineffective and is not recommended. This recommendation is based on lower mass loading for liquid injections compared to solid application, limited radius of influence, lower persistence and possible density-driven effects.
Table 2. Site Conditions to Choose an Appropriate Sulfate Delivery Method
| Sulfate Delivery Method | Preferable Site Conditions | Advantages | Limitations |
| Land application | Open, unpaved land surface free of obstruction. Permeable geological materials. Shallow depth to groundwater (up to 15 ft. bgs). Significant natural precipitation (or easy access to source of irrigation water). | Potential benefit to entire plume footprint and vadose zone impacts.1 Simplicity of application and reapplication (surface broadcasting, no drilling required). Groundwater fluctuations do not impact delivery method. | Uncertainty about sulfate breakthrough to groundwater. Possibility of sulfate consumption or retention in vadose zone. Additional irrigation required if natural precipitation is insufficient. |
| Permeable filled borings (PFB) (gypsum and gravel) |
Primarily saturated zone hydrocarbon impacts. Surface access for drilling. Well-defined groundwater flow velocity and direction. | Effective sulfate delivery to groundwater. Provides sustained source of sulfate (longevity >5 years). Can respond to fluctuating groundwater table. Can treat deeper impacts and beneath confining layers. | Requires multiple soil borings for PFB due to limited radius of influence. Reapplication at same PFB location not feasible. |
| Permeable trench (gypsum and gravel) |
Permeable geological materials. Land free of obstruction. Shallow depth to groundwater (up to 15 ft. bgs). | Effective sulfate delivery to groundwater. High longevity (> 5 years).Can respond to fluctuating groundwater table. Continuous sulfate curtain upgradient and / or within source and plume area. | Trenching required. Reapplication within the same trench not feasible. Limited to shallower groundwater-related impacts. |
| Excavation backfill | Excavated sites where residual impacts around and beneath the excavation still remain. Intersecting groundwater, significant natural precipitation or easy access to source of irrigation water. | Provides sustained source of sulfate (longevity > 5 years).Easy to apply during excavation activities. Potential benefit to groundwater plume and possibly to vadose zone impacts that may remain below the excavation. | Feasible only at sites undergoing some degree of excavation. Application limited to the practical depth of excavation. Emplaced sulfate may not intersect groundwater, depending upon depth of excavation and groundwater depth. |
3. Implementation of Sulfate Delivery Methods
While overlaps exist in the possible site settings and benefits of the sulfate delivery methods, each method also has some unique features that need to be considered during the design, implementation, and monitoring phases. Table 3 presents guidance on implementation for these methods.
Table 3. Summary Guidance on Implementation of Sulfate Delivery Methods
| Sulfate Delivery Method | Key Considerations |
Land Application (granular agricultural gypsum)  |
Select the land application footprint around the appropriate monitoring wells. Broadcast agricultural gypsum3 on the land surface at the rate between 0.6 to 1.2 lb./ft2 (track mounted spreader or fertilizer broadcaster).Till the top 4 to 6 inches of soil to mix in gypsum. Create a 4 to 6 inches high berm with soil or haybales (to minimize surface run-off to areas outside the target area).If possible, time the land application when ground is already wet (during snow melt) to enable gypsum to dissolve naturally (without artificial irrigation). If artificial irrigation is needed, estimate irrigation volume based on monthly maximum precipitation intensity. |
Permeable Filled Borings (granular agricultural gypsum-gravel) 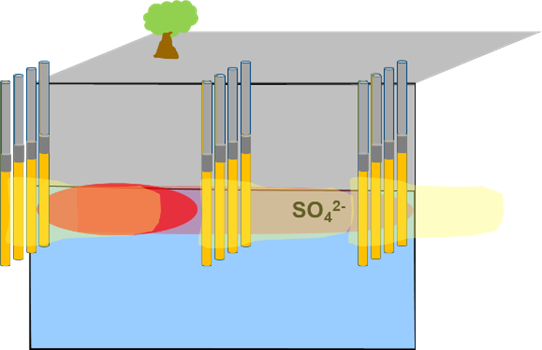 |
Can be placed upgradient of source, as well as to intersect the dissolved plume. Drill borings at the closest spacing and the largest diameter feasible. Pour 1:1 mix ratio of granular agricultural gypsum and gravel (proppant) into the soil borings. (Note: Consider using a proppant with a similar density/particle size as gypsum, such as rhyolite or pea gravel, that can help prevent material segregation in borehole).Cap with two feet of bentonite and cement to ground surface. |
Permeable Trench (granular agricultural gypsum-gravel) 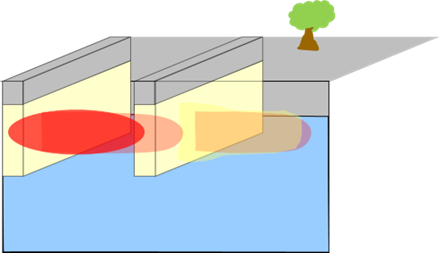 |
Can be placed upgradient of source, and/or to intersect the dissolved plume. Advance a trench up to the depth of groundwater impacts. The length of the trench will be determined by width of the source area or plume and other site constraints. Place 1:1 mix ratio of granular agricultural gypsum and gravel (proppant). Mixture would be placed up to the depth of shallowest groundwater depth to account for water table fluctuations. (Note: Using a proppant with a similar density as gypsum, such as rhyolite, can help prevent material segregation). Cap with approximately two feet of bentonite and cement to ground surface. |
Excavation Backfill (granular ag gypsum) 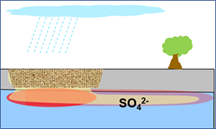 |
Add agricultural gypsum (one percent w/w of the excavation backfill). This quantity could be uniformly mixed with the backfill or placed as a layer at the bottom of the excavation. Dissolution and transport of sulfate could be achieved by infiltration, intersecting groundwater, or through artificial irrigation. |
4. Monitoring and Analytical Plan
Monitoring is an important element prior to designing the remedy and for assessing performance over time. It should be conducted considering the expected changes that will likely occur in the target analyte(s) (e.g., benzene, sulfate) in accordance with the redox stoichiometric equation. The arrows indicate the direction of change for concentrations and isotopic shifts.
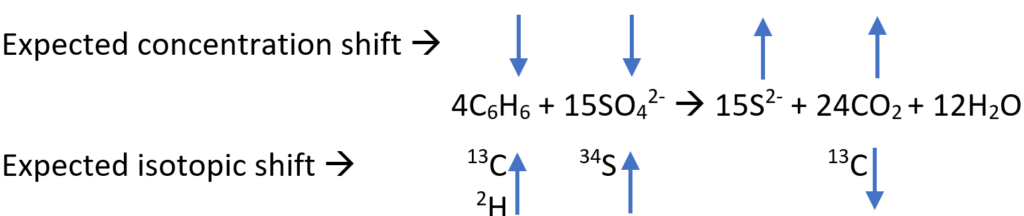
For concentration shifts, a down arrow means concentration of the identified constituent is expected to decrease and an up arrow means concentration is expected to increase as reaction proceeds. For isotopic shift, a down arrow means the constituent is expected to be depleted in the heavier isotope and an up arrow means it is expected to be enriched in the heavier isotope as reaction proceeds.
Table 4 provides example analytical data that can be used for pre-baseline monitoring (to determine if sulfate addition is suitable), baseline monitoring before applying sulfate, and post-delivery performance monitoring. Bromide salt (e.g., CaBr2) is sometimes added along with gypsum to use bromide as a conservative tracer to track infiltration and delivery of applied salts. In addition, it is recommended to collect 34S in SO42- data for the gypsum used as a source of sulfate. This can be done by adding a small quantity of gypsum salt into a sample jar filled with distilled water and submitting the resulting water sample for 34S in SO42-analysis.
The primary line of evidence would be a (log-linear) concentration-time series plot such as Figure 1. Compare the trends before and after sulfate addition.
Table 4. Sample Analytical Plan
| Analytes | Example Method | Preliminary assessment | Baseline | Performance Monitoring | Expectations |
| Bromide, Nitrate, Sulfate | EPA 300.0 / 9056A | X | X | X | Sulfate concentration is depleted at baseline and increases upon sulfate addition. Bromide used for breakthrough assessment. Nitrate for general geochemistry. |
| Iron | SW-846 6010B / 6020 | X | X | Iron II concentration may decrease after sulfate addition as precipitation with sulfide occurs. | |
| Dissolved Inorganic Carbon (DIC) | 5310C | X | X | X | DIC may increase upon sulfate addition but is generally expected to be buffered. |
| Methane | RSK-175 | X | X | X | Helps assess methanogenesis at baseline and change in methane concentration following sulfate addition. |
| Sulfide | EPA 9034 | X | X | Sulfide is stoichiometrically expected to increase after sulfate addition but is precipitated out with iron. Therefore, it is expected to be low or non-detect. | |
| 34S in SO42- | Specialty Isotope Lab | X1 | X | Signature is enriched after sulfate addition compared to source sulfate signature. | |
| 13C in DIC | Specialty Isotope Lab | X | X | Signature is depleted compared to baseline condition as mineralization of petroleum hydrocarbons occurs. | |
| BTEX / TPH | EPA 8260 / 8015 | X | X | X | Petroleum hydrocarbon concentrations are expected to trend downwards after sulfate addition. |
| 2H in Benzene and / or 13C in Benzene | Specialty Isotope Lab | X | X | Signature is expected to be enriched following sulfate addition compared to baseline or control. 2H-benzene is usually more responsive than 13C-benzene. |
References
J.A. Cunningham, H. Rahme, G.D. Hopkins, C. Lebron and M. Reinhard, 2001. Enhanced in situ bioremediation of BTEX-contaminated groundwater by combined injection of nitrate and sulfate. Environmental Science & Technology 35, no. 8: 1663-1670
R. Kolhatkar and M. Schnobrich, 2017. Land Application of Sulfate Salts for Enhanced Natural Attenuation of Benzene in Groundwater: A Case Study, Ground Water Monitoring & Remediation 37, no. 2, 43-57, Open Access: https://doi.org/10.1111/gwmr.12209
T. Buscheck, D. Mackay, C. Paradis, R. Schmidt, and N. de Sieyes, 2019. Enhancing Microbial Sulfate Reduction of Hydrocarbons in Groundwater Using Permeable Filled Borings, Ground Water Monitoring & Remediation 39, no. 3, 48-60, Open Access: https://doi.org/10.1111/gwmr.12346
K.S. Sra, V. Ponsin, R. Kolhatkar, D. Hunkeler, N.R. Thomson, E.L., Madsen, and R. Buscheck, 2022a. Sulfate Land Application Enhances Biodegradation in a Petroleum Hydrocarbon Smear Zone, Ground Water Monitoring & Remediation 43, no. 1, 44-59, Open Access: https://doi.org/10.1111/gwmr.12547
K. Sra, R Kolhatkar, D. Segal, and J. Wilson, 2022b. Sulfate Delivery Methods for Enhancing Biodegradation of Petroleum Hydrocarbons, National Tanks Conference, Pittsburgh, Sep 2022.
